The COVID-19 pandemic has acted as a catalyst, propelling significant changes in diagnostic pathways and the adoption of transformative technologies. This seismic shift is not just about overcoming immediate challenges but signifies a fundamental transformation in the landscape of diagnostics across Europe and beyond. The convergence of innovative technologies and patient-centric service delivery models is reshaping care pathways, leading to a decentralisation of diagnostic services and an evolution in healthcare paradigms.
This pandemic-induced metamorphosis heralds an era where diagnostics take centre stage, unlocking unprecedented scope and capacity. It goes beyond crisis management, presenting opportunities to drive economic growth and optimise healthcare costs. For patients, healthcare systems and molecular diagnostic supplies alike, the recognition that improved access to diagnostics, spanning point-of-care testing, self-testing kits, genomic testing, digital diagnostics, and AI-based analytical solutions, is pivotal in meeting the escalating demand for healthcare. The end goal? To bolster the productivity of healthcare systems
The Precision Medicine Paradigm
The concept of personalised medicine was originally conceived with the aim of tailoring medical treatments to align with the distinctive characteristics of individual patients. This envisioned a shift in the clinical treatment paradigm, moving away from a trial-and-error approach towards ensuring “the right drug, for the right patient, at the right time.” In the contemporary landscape, a convergence of factors, including public investment, advancements in biotechnology, and the digitisation of health, has propelled personalisation beyond the confines of therapy selection. It now extends its influence into drug discovery, the planning and delivery of care, and notably, the ways in which businesses and consumers alike interact with companies committed to enhancing health outcomes.
When integrated into healthcare, precision medicine has the potential to yield more precise diagnoses, predict disease risk before symptoms occur, and design customised treatment plans that maximise safety and efficiency – a feat that serves to challenge healthcare’s poor track record of productivity gains.
Molecular Diagnostics: Fuelling the Precision Medicine Engine
The driving forces behind precision medicine’s transformative potentials include progress in diagnostics, digital devices, and imaging, coupled with a sophisticated array of analytics tools collaboratively employed across diverse institutions and stakeholders.
Molecular diagnostics serves as the data powerhouse that fuels the precision medicine engine. The detailed insights provided by molecular diagnostics enable healthcare professionals to move beyond broad treatment approaches, offering a tailored and precise roadmap for patient care.
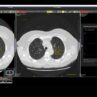
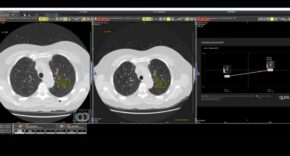
In the ever-evolving realm of molecular diagnostics, significant strides have been made, inaugurating a new period of precision healthcare. The identification and quantification of biomarkers, akin to measurable beacons of biological processes, have seen noteworthy advances. Key players in this transformative journey, including Real-time quantitative polymerase chain reaction (qPCR) and microarray analysis – concomitant with no shortage of molecular diagnostics suppliers – have redefined disease diagnosis, prognosis, and treatment monitoring. This intricate dance of innovation not only equips clinicians with the knowledge to make informed decisions on targeted therapies but also optimises treatment outcomes.
The qPCR Advantage
In recent decades, qPCR has gradually supplanted several culture methods. Departing from reliance on cytological (cellular analysis) or antigen-based information for pathogen identification, qPCR strategically amplifies distinctive DNA fragments specific to a pathogen’s genome. Fluorescently tagged probes, designed to complement the DNA in question, undergo multiple rounds of heating in a thermocycler when mixed with the sample. In the presence of the target pathogen’s DNA, amplification occurs, releasing fluorescent markers from the probes, resulting in an exponential rise in fluorescence for pathogen-positive samples.
This method stands out for its accuracy and speed. Traditional methods, fraught with limited sensitivity and time-consuming procedures, have allowed powerful tools under the umbrella of such nucleic acid amplification tests as PCR to become molecular diagnostic medallists. This arsenal of techniques facilitates rapid and accurate pathogen identification, enabling early diagnosis and elevating patient care. The broader impact extends to effective epidemiological surveillance, with timely outbreak investigations and monitoring of antimicrobial resistance, thereby fortifying global public health – and streamlining diagnostics for faster and more accurate result generation.
Alongside this, novel technologies such as digital PCR (dPCR) and next-generation sequencing (NGS) have spawned to overcome the intrinsic limitations of “classical” qPCR. Indeed, dPCR is considered the third generation of PCR. It was developed to overcome certain limits of conventional amplification techniques, particularly to allow detection of small amounts of target nucleic acids.
Yet real-time quantitative RT-PCR (RT-qPCR, also RQ-PCR) remains the current gold standard, most notably in the realm of oncology molecular where monitoring using BCR-ABL RQ-PCR is a necessary laboratory technique for monitoring the efficacy of tyrosine kinase inhibitor therapy and quantitatively assessing minimal residual disease. A stumbling block to dPCR remains its limited availability and absent standardisation – despite the promise it potentiates; not least its higher sensitivity, high detection capabilities – as little as 1 copy of BCR-ABL1 transcript – and its ability to perform an absolute quantification of the target without the need for a standard curve.
The Promise of Biomarkers: A Revolution in Diagnosis, Prognosis, and Treatment
Going beyond oncology, the pursuit of medical precision has unveiled a litany of biomarkers that have emerged as invaluable allies in decoding biological nuances. RQ-PCR and microarray analysis, pivotal in identifying and quantifying specific such biomarkers, have led to the development of companion diagnostic tests. This paradigm shift is revolutionising disease diagnosis, prognosis, and treatment monitoring. As clinicians unravel the intricate tapestry of biomarkers, molecular diagnostics emerges as a potent force, empowering them to discern patients most likely to benefit from targeted therapies, thereby optimising treatment outcomes.
Beyond PCR, the repertoire of molecular diagnostics techniques has staged a paradigm shift in the realm of infectious disease detection and surveillance. Indeed, a striking innovation within molecular diagnostics is the advent of point-of-care testing (POCT), heralding a revolution in on-site diagnostic capabilities. These molecular POCT devices, with their compact form factors and user-friendly interfaces, leverage cutting-edge techniques like isothermal amplification and microfluidics. This empowerment enables immediate diagnosis and treatment decisions at the point of care, manifesting transformative potential, particularly in resource-limited settings, remote locations, and emergency situations. A compelling illustration of molecular diagnostics in action is seen in the research focused on developing diagnostic methods for African swine fever virus (ASFV) and classical swine fever virus (CSFV), both substantial threats to the pig industry. Researchers have ingeniously introduced Recombinase-aided amplification (RAA) technology combined with a nucleic acid test strip (RAA-strip) for specific detection, showcasing high sensitivity and specificity, even at low levels of viral DNA/cDNA.
Diagnostics companies face the imperative of defining their role in the future of health, strategically positioning themselves in emerging fields. Placing diagnostics at the core of disease and patient pathways emerges as a strategic imperative. Early disease detection, accurate guidance for treatments, and improved cost-effectiveness are pivotal not just for the industry but, crucially, for enhancing patient outcomes. As we navigate this diagnostic revolution, the decisions made today will shape the future of healthcare tomorrow.
Author: Hidaya Aliouche