UK medical devices company, Sky Medical Technology Ltd (Sky), has received U.S. Food and Drug Administration (FDA) 510(k) clearance to market its unique bioelectronic device to prevent life-threatening blood clots in non-surgical patients at risk for venous thromboembolism (VTE).
The clearance adds to previous 510(k) clearance for the use of geko™ device in increasing immediate post-surgical VTE prevention, making the device the first bioelectronic muscle pump activator of its kind to be cleared by the FDA for VTE prevention across all patients, including non-surgical patients.
VTE is a deadly risk to hospitalized patients, particularly those who are immobile as a result of acute stroke or trauma recovery. Estimates suggest that 60,000 – 100,000 Americans die each year as a result of the condition, with 10 to 30% of patients dying within one month of diagnosis. In England the estimate is 40,000 deaths annually.
This expanded FDA clearance unlocks access to a $3.5bn1 USA VTE prevention market for Sky.
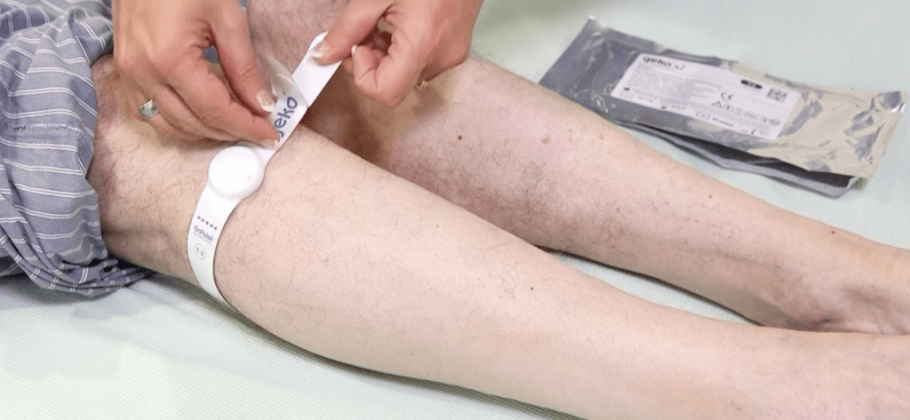
The geko™ is an easy to use, battery powered, wearable therapy device. The size of a wristwatch and worn at the knee, the daily disposable device gently stimulates the common peroneal nerve activating the calf and foot muscle pumps, resulting in increased blood flow in the deep veins of the calf. It operates without external pressure to the leg and allows complete mobility.
A study of the geko™ device to prevent VTE in acute stroke patients reported zero VTEs in patients wearing the geko™ device device alone, compared to 11 VTEs in patients prescribed intermittent pneumatic compression (IPC). The data has driven rapid adoption across multiple NHS trusts and international markets, as well as this expanded FDA clearance.
The investigators, led by Dr. Indira Natarajan, consultant stroke physician and clinical director for neurosciences and Professor Christine Roffe at the NHS Royal Stoke University Hospital, also determined that 30% of patients are contraindicated or became intolerant to IPC (discomfort or dislike to IPC). It is to this unmet need cohort that the geko™ device was fitted, reporting a zero VTE incidence and good patient tolerance. The data has driven rapid adoption across multiple NHS trusts and international markets, as well as the expanded FDA clearance.
Dr Natarajan, a consultant stroke physician at Royal Stoke University Hospital who led the audit, said: “The real-world data has shown a need to use the geko™ device when other VTE prophylaxis strategies are contraindicated or impractical and provides an option where previously patients would have had no other intervention available to them. The geko™ device is now in routine use at the Royal Stoke and has marked significant change to our practice.”
Commenting on the FDA clearance, Sky CEO Bernard Ross said: “This latest 510(k) builds on our previous FDA indications to address life threatening blood clots and complications related to swelling after orthopaedic surgery, conditions experienced by more than 1 million US patients with unmet need every year.
“Our bioelectronic medicine therapy, OnPulse™, embedded in the geko™ device, completely redefines the way vascular related conditions are treated. Through our innovative mechanism of neuromuscular electrostimulation, we are the first clinically proven bioelectronic compression technology to prevent and treat a wide range of acute and chronic circulatory conditions both in the US, at home in the UK and internationally. New care pathways are in development and we plan to submit further FDA applications to expand our claims.”