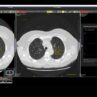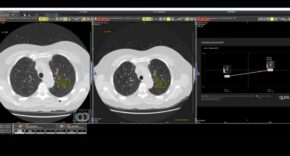
- Multi-centre randomised self-controlled trial comparing standard of care (SoC) with and without the geko™ device in patients with hard-to-heal venous leg ulcers (VLUs), is published in the International Wound Journal.
- The study results show the geko™ device, combined with multi-layer compression, doubles the rate of healing vs. compression alone1.
- VLUs affect one in 500 adults in the UK2, costing the national healthcare system around £2 billion annually3.
UK-based medical device manufacturer Sky Medical Technology today announces the International Wound Journal has published the company’s landmark multi-centre randomised self-controlled trial (RCT).
The study1 compared standard of care (SoC) with and without the geko™ device in patients with hard-to-heal venous leg ulcers (VLUs) and reports an acceleration in the rate of healing by more than double in patients treated with the geko™ device vs. SoC alone. The results offer new hope to patients suffering long-standing chronic wounds.
The study is the first RCT to show a statistically significant increase in the rate of healing of VLUs treated with an advanced neuromuscular electrostimulation device (the geko™ device), adjunctive to SoC.
Approximately 3.8 million adults in the UK suffer with a wound4. Many are hard-to-heal and despite following best practice wound healing can be prolonged or often never achieved, with over 50 percent of VLUs failing to heal in 12 months4. Patients experience pain, anxiety, altered body image and isolation, leaving many without hope of improvement, suffering wound infections and recurrence.
The financial burden of non-healing wounds on hard pressed healthcare systems is also huge, higher than for cancer and cardiovascular disease. The annual NHS cost to manage wounds is £8.3 billion annually, £5.6 billion of which is the management of wounds that fail to heal4. VLUs affect one in 500 adults in the UK costing the NHS over £2 billion annually2, 50 percent of which remain unhealed4 – a number that is set to rise5.
Agnes Juguilon Collarte, Tissue Viability Specialist Nurse Lead, Inner Northwest Division (Central London, Hammersmith & Fulham and West London), comments: “As a clinician in wound care, especially when managing patients with chronic wounds, the ultimate goal is improvement in healing rates. The results of this randomised self-controlled trial are extremely impressive and are also borne out in our direct experience of real-world use. Non-healing VLUs stop patients living their lives and robs them of hope. The geko™ device consistently accelerates VLU healing in the patients I treat.”
Bernard Ross, Founder and CEO at Sky Medical Technology, comments: “We are delighted to have completed this landmark study which further validates geko™ device effectiveness in reporting a doubling of the rate of VLU healing, in a market that has seen surprisingly little real innovation in SoC over 20 years. Gold standard multi-layer compression can be effective, particularly in less complex and smaller wounds, but even in these patents is too often associated with low patient adherence. The success of the geko™ device in increasing the rate of SoC VLU healing is a critical step in bringing MedTech innovation to VLU patients around the world and to modernising standard of care – and cements our commitment to the streamlining of wound-care through evidence-based clinical efficiency for better patient outcomes.”
The geko™ device is a small-in-size, self-adhesive, wearable neuromuscular electrostimulator that is applied to the surface of the skin just below the knee, over the head of the fibula bone. It delivers a gentle electrical pulse, once per second to the common peroneal nerve, activating the calf and foot muscle pumps, increasing venous, arterial, and microvascular flow, effectively replicating the effects of exercise.6
References
- Bull, RH, Clements, D, Collarte, AJ, Harding, KG. The impact of a new intervention for venous leg ulcers: A within-patient controlled trial. Int Wound J. 2023; 1- 9. doi:10.1111/iwj.14107 https://onlinelibrary.wiley.com/doi/10.1111/iwj.14107
- https://www.nhs.uk/conditions/leg-ulcer/
- Phillips, CJ, Humphreys, I, Thayer, D, et al. Cost of managing patients with venous leg ulcers. Int Wound J. 2020; 17: 1074– 1082. https://doi.org/10.1111/iwj.13366
- Guest JF, Fuller GW, Vowden P. Cohort study evaluating the burden of wounds to the UK’s National Health Service in 2017/2018: update from 2012/2013. BMJ Open. 2020 Dec 22;10(12):e045253. doi: 10.1136/bmjopen-2020-045253. PMID: 33371051; PMCID: PMC7757484.
- Probst, S., Weller, C.D., Bobbink, P. et al. Prevalence and incidence of venous leg ulcers—a protocol for a systematic review. Syst Rev 10, 148 (2021). https://doi.org/10.1186/s13643-021-01697-3
- Tucker A, Maass A, Bain D, Chen LH, Azzam M, Dawson H, et al. Augmentation of venous, arterial and microvascular blood supply in the leg by isometric neuromuscular publication of the International College of Angiology, Inc. 2010 Spring; 19(1): e31-7.
- Godwin M, Ruhland L, Casson I, MacDonald S, Delva D, Birtwhistle R, Lam M, Seguin R. Pragmatic controlled clinical trials in primary care: the struggle between external and internal validity. BMC Med Res Methodol. 2003 Dec 22;3:28. doi: 10.1186/1471-2288-3-28. PMID: 14690550; PMCID: PMC317298.